
In the lewis structure of BrCl3, we find that each chlorine atom has 8 valence electrons thereby satisfying its octet. Step 5: Now we will predict and draw the lewis structure of BrCl3 as shown below. Step 4: Lastly, we will determine the central atom which is usually the single atom present in the molecule. Step 3: Next, we will find the total number and the type of bond formation between the atoms involved.įor BrCl3, a single shared covalent bond for each is formed between the bromine atom and the 3 chlorine atoms. For bromine it is 1electron and for 3 chlorine atoms, it is 1 electron each to obtain an octet. Step 2: Secondly, we will determine the electrons deficient in each atom to attain a stable electronic configuration. Hence, total number of valence electrons in BrCl3 = valence electron in bromine + valence electrons in 3chlorine atoms We know that both bromine and chlorine belong to group 17 of the periodic table having 7 electrons each in their valence shell. Step 1: Firstly, let us determine the total number of valence electrons in BrCl3.
#Brf5 molecular geometry series
Let us follow a series of steps that will ease our understanding of the methodology to draw lewis structure of BrCl3:
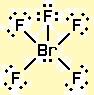
It helps us in determining bonded and non-bonded electrons.įor BrCl3, the lewis structure reveals that a total of 28 valence electrons(7 from bromine + 7(3) from 3chlorine atoms) are required to form a single BrCl3 molecule. The lewis structure gives a simplified representation of the valence electrons present across an atom in a molecule. Atoms usually having lower than 8 electrons react to form more stable compounds to complete their octet.Īn exception to the octet rule is hydrogen which requires only 2 electrons to become stable attaining the Helium gas configuration. Octet rule refers to the tendency of atoms to have 8 electrons in their valence shell or attain the nearest noble gas configuration. Hence the valence electrons in chlorine are 7. We see that in the outmost shell the number of electrons is 7(in 3s and 3p). To understand the valence electrons we need to write the electronic configuration of the atom from which we can easily find the number of valence electrons in the atom.įor example: Take chlorine with atomic number 17.Įlectronic Configuration = 1s22s22p63s23p5
These valence electrons help in the bond formation by getting accepted or donated by other species. Valence electrons of an atom are those electrons that are present in the outermost shell and take part in bond formation.ĭuring bond formation, it is required to donate or accept electrons in order to fulfil their octate.


 0 kommentar(er)
0 kommentar(er)
